REIG JOFRE
PHARMACEUTICAL COMPANY REIG JOFRE
- Location: SANT JOAN DESPÍ, BARCELONA.
- Client: LABORATORIO REIG JOFRE, S.A.
- Services:PROJECT AND SITE MANAGEMENT
- Area: 2.943 m2
- Year: 2018 – 2021
To achieve all this, Indus drew up the project, carried out the work relating to the grant of the environmental licence and managed the site.
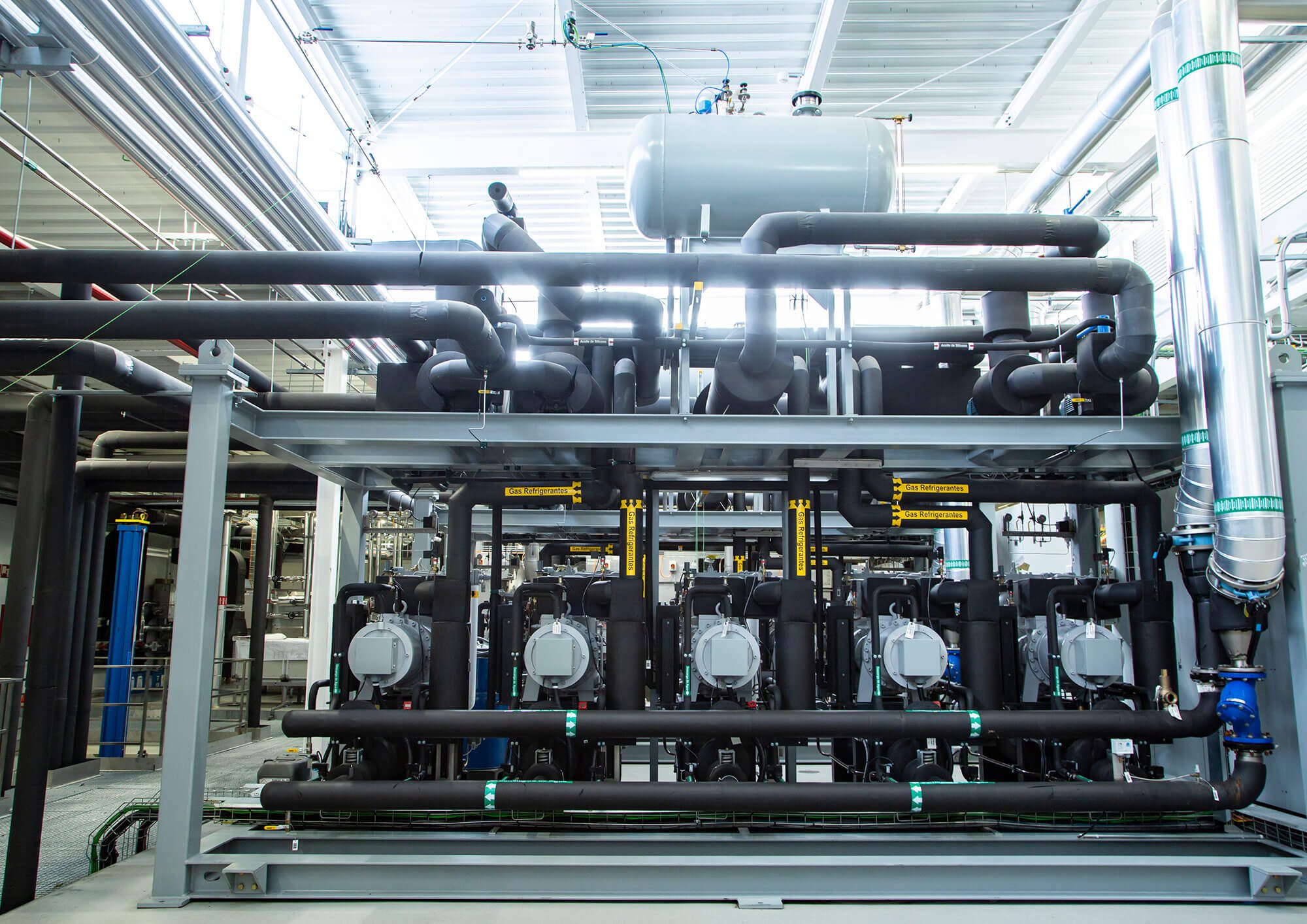
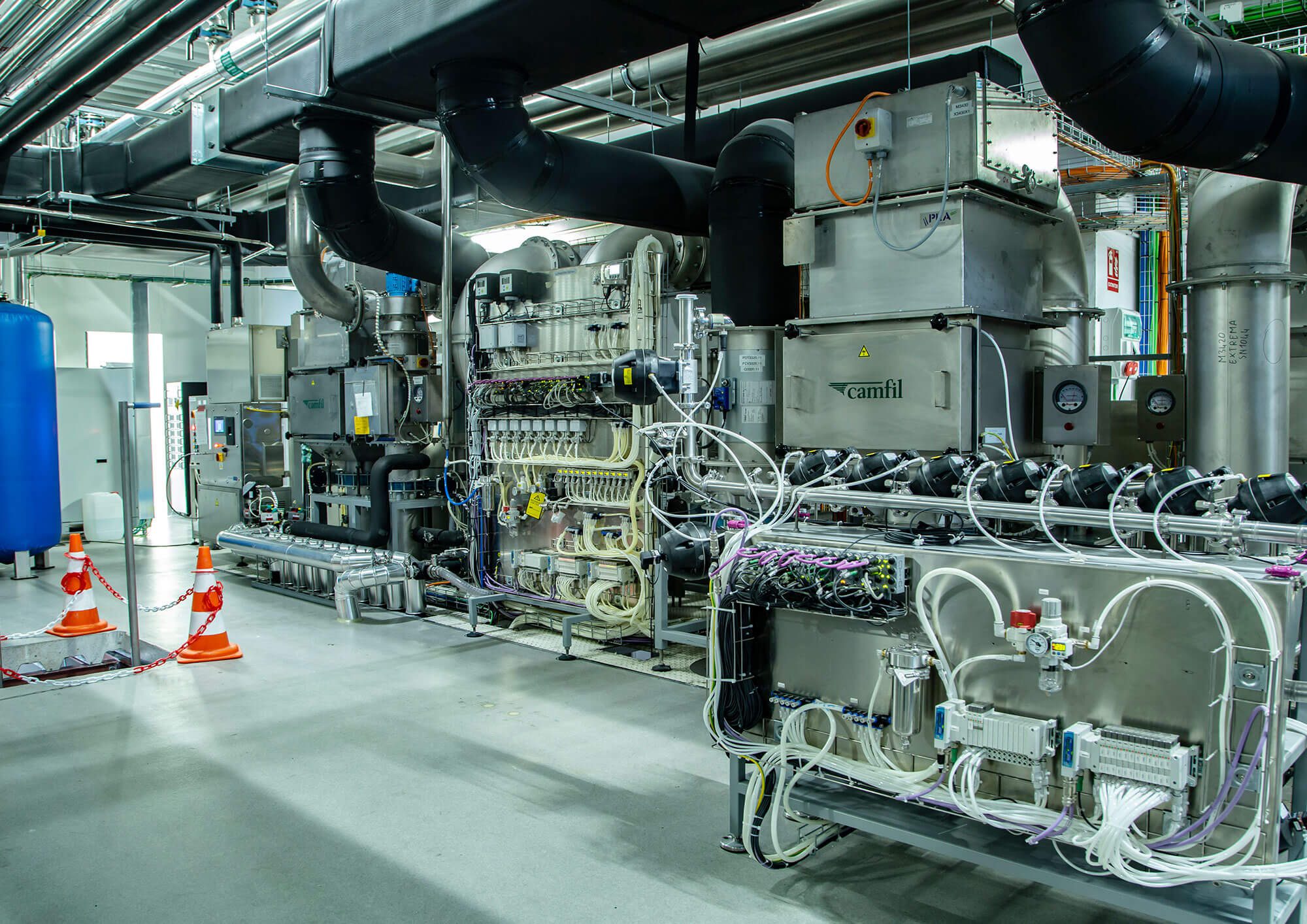
The injectable vial manufacturing area, built in 2019, was remodelled and extended during the first half of 2021. This was done so that sterile products with strict negative temperature control requirements, such as SARS-CoV-2 vaccines, could be manufactured there.
Following the conclusion by the pharmaceutical company Reig Jofre of an agreement to produce the Janssen Covid-19 vaccine in its new injectable plant, and after the completion of the technology transfer process, production started in the second half of 2021.
In this project for the injectable plant, INDUS was in charge of planning the following actions:
– Transforming the warehouse for finished products into a cold storage warehouse. A suspended floor with mechanical ventilation was built, and new cold storage rooms were installed above it: one with a surface area of 250 m² for storing items at -20°C, and one with a surface area of 100 m² for storage at -40°C. In addition, loading/unloading antechambers were built for both of them.
– Working on existing production areas to turn them into cold storage rooms for storing intermediate products. Two walk-in freezers were built: one with a surface area of 168 m² for storing items at +2/+8°C; and a 172 m² one with a 20 m² antechamber, for storing items at -20°C.
– The production area was extended through the addition of a new 572 m² first floor for an ultra-low temperature freezer room, a defrosting room, a room for waste from packaging, and a technical office. The new floor was built with a metal structure, concrete composite slabs, hygienic closing systems with pharmaceutical-grade sandwich panels, and continuous PVC flooring. In addition, an external metal platform was built for the condensing units of the HVAC systems and new walk-in freezers.
– A new 1,500 kg hydraulic goods lift was installed to move materials more easily between the general warehouse on the ground floor and the new first floor. This will be used to take raw materials to the ultra-low temperature freezer area.












Central Barcelona
Via Augusta, 4, 08006 Barcelona
Teléfono: 932 17 56 54
Delegación Madrid
Santa Úrsula, 7. 28801,
Alcalá de Henares, Madrid
Teléfono: +34 911 177 221
Copyright @ INDUS: Engineering, Architecture and Consulting - Legal Warning - Privacy Policy - Cookies Policy